March 2, 2022
Small cyberphysical watermarks could prevent huge headaches caused by fake meds
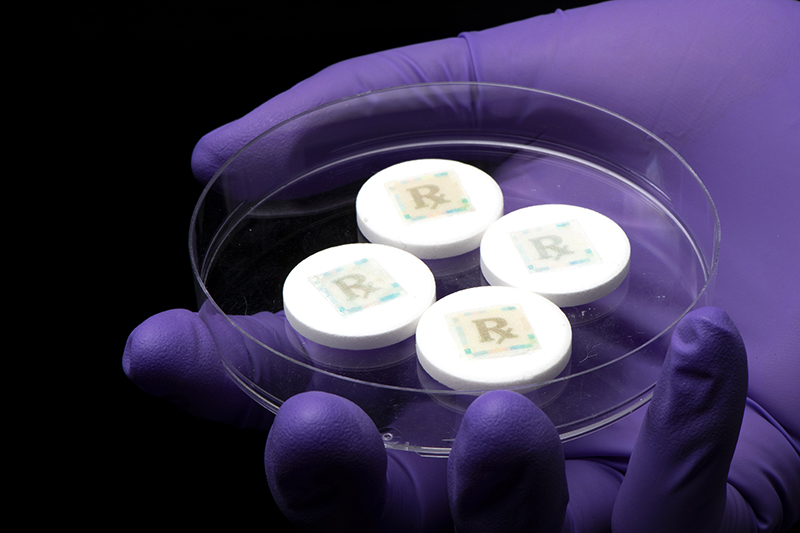
Counterfeit medications and pharmaceutical products are just a click away from being purchased from online pharmacies via smartphone.
However, new anticounterfeiting technology can turn a smartphone into a lifesaver by simply taking a picture of a cyberphysical watermark and confirming if the medication is real or not.
The technology was developed by a team of biomedical engineers from Purdue University and was published in journal Advanced Functional Materials.
Young Kim, associate head for research and an associate professor in Purdue’s Weldon School of Biomedical Engineering, says the continued rise of counterfeit medications, pharmaceutical products and medical supplies can be attributed to the increase of online pharmacies, many of which are unregulated.
To address the growing issue, the U.S. Food and Drug Administration is requiring by 2023 that medications have unit-level traceability through the Drug Supply Chain Security Act. Kim said pharmaceutical companies have the ability to track boxes or sheets of medications, but adding traceability directly on a pill could require adding numerous manufacturing and data management steps.

“We have technology that can empower patients to check to see if the cyberphysical watermark on the medications they are taking is real or counterfeit,” Kim said. “This allows them to also confirm dose, frequency and information on the medicine.”
“A paper watermark is commonly used on currency and a passport to discourage counterfeiting, and we are affixing a watermark on an individual medicine that is readable by a smartphone camera to extract a hidden digital key,” Kim said. “Purdue has an excellent track record of watermarking and inkjet printing research. We are proud that we have extended such national security research into pharmaceuticals as counterfeit medicines are a national security problem.”
The cyberphysical watermark is printed on specialized fluorescent silk with FDA-approved food dye through an inkjet printer – a common technique that bakers use for placing edible photos on cakes.
Hee-Jae Jeon, a postdoctoral fellow who is also the first author of the study, said that silk is all protein and can be edible.
“Silk is a great choice for eating, as we also were not wanting to use synthetic or artificial materials and fluorescent silk makes a counterfeiter very difficult to duplicate the watermark.”
Jung Woo Leem, a postdoctoral research associate, said that in addition to being edible, the silk is a good option for pharmaceuticals because engineers have the ability to change the biopolymer’s shape, structure and flexibility.
The engineers also addressed how to use the technology with different smartphone models, photo quality and light.
“A person can take a photo under different light conditions and will have different images. It’s the same issue when patients take photos of our watermark in their phone,” Kim said. “The reference colors on the watermark’s periphery allow us to know the true color value of the watermarked image as each smartphone has different spectral sensitivity.”

Placed on pills using a simple sugar glue, the smallest size of watermark the team could produce is 5 milimeters by 5 milimeters. While the team has had success with solid pills, it is working also on developing technology for liquids.
Kim said that the technology could be used first on name-brand medications and restricted narcotics before being rolled out on over-the-counter medications and generics.
This work continues the research Kim and his team have done on medication security.
In addition to Kim, Jeon and Leem, members of the research team were Yuhyun Ji and Sang Mok Park from Purdue’s Weldon School of Biomedical Engineering; and Jongwoo Park, Kee-Young Kim and Seong-Wan Kim from the Department of Agricultural Biology at the National Institute of Agricultural Sciences in South Korea.
Funding came from the Cooperative Research Program for Agriculture Science and Technology Development (PJ015364) from the Rural Development Administration of the Republic of Korea, the U.S. Air Force Office of Scientific Research (FA2386-17-1-4072), the Trask Innovation Fund from Purdue University, and the NIH Technology Accelerator Challenge from the National Institutes of Health. The technology was disclosed to the Purdue Research Foundation Office of Technology Commercialization, which has applied for a patent from the U.S. Patent and Trademark Office to protect the intellectual property. For information on licensing opportunities, contact Patrick Finnerty of OTC at pwfinnerty@prf.org about 2022-KIM-69723.
Writer, Media contact: Matthew Oates, 765-586-7496 (cell), oatesw@purdue.edu, @mo_oates
Sources: Young Kim, youngkim@purdue.edu
Hee-Jae Jeon, jeon86@purdue.edu
Jung Woo Leem, leem0@purdue.edu
Recent Purdue Biomedical Engineering stories
A horse slicker is just a horse slicker, of course, of course, unless it can monitor chronic disease
Enhancing ordinary items for addressing health outcomes
Cracking the code of cellular defense
ABSTRACT
Cyber-Physical Watermarking with Inkjet Edible Bioprinting
Hee-Jae Jeon, Jung Woo Leem, Yuhyun Ji, Sang Mok Park, Jongwoo Park, Kee-Young Kim, Seong-Wan Kim, and Young L. Kim
Counterfeit medicines are a fundamental healthcare problem, threatening patient safety and public health as well as causing economic damage. Online pharmacies and the ongoing pandemic have promoted medicine counterfeiting. However, the existing anticounterfeit methods are limited because of material toxicity, low security, and complicated fabrication. Here a dosage-level security method is introduced that combines digital watermarking and physical printing at the material level. A set of requirements is designed to ensure the edibility, printability, imperceptibility, and robustness of cyber-physical watermarking. An inkjet printer using safe food coloring is adapted to print a watermarked image on a recombinant luminescent silk protein taggant to enhance attack resistance. Machine learning of color accuracy recovers unavoidable color distortions during printing and acquisition, allowing robust smartphone readability. An edible watermarked taggant affixed to each individual medicine can offer anticounterfeit and authentication features at the dosage level, empowering every patient to aid in abating illicit medicines.
